secondary
Making the World Around Us
Atoms are tiny objects that make up the world around us. They are about a hundred thousand times smaller than a human hair and way too small to be able to see! Atoms come in different types, which we call Elements: Oxygen, Gold and Carbon are all examples of different Elements.
For a long time, scientists believed that Atoms were the smallest, simplest objects that existed, but now we know that atoms are actually made up of three even smaller objects - Protons, Neutrons and Electrons.
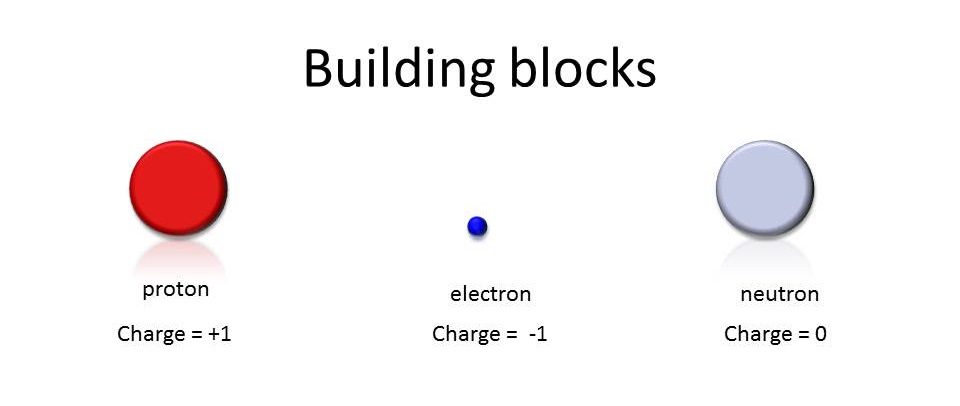
These three particles each have a different role in the Atom. The Protons and Neutrons are found in the nucleus at the centre of the Atom. Protons have a positive charge, and repel each other. It is difficult to get Protons too close to one another, so Neutrons are packed in between the Protons. Neutrons are neutral and have no charge. The role of the Neutrons is to stop the Protons from getting too close to one another. Because Protons are positive, they will repel other positive Protons; the Neutrons are added into the Nucleus to keep the Protons further away from each other so that the entire nucleus is not ripped apart.
The number of Protons in an Atom tells us what type of Element it is – every element will have a different number of Protons. If you add or remove a Proton from an Atom, then it will become a different Element. Neon has 10 Protons and is a gas; add one more Proton and you will form Sodium, which is a metal.
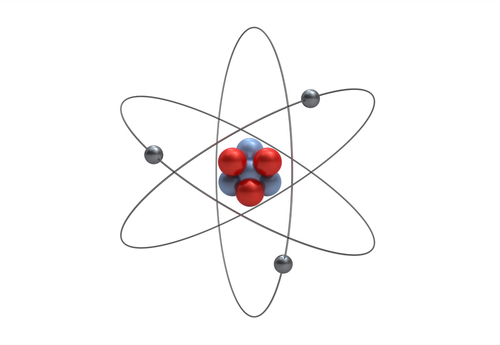
In the picture below, you can see Protons and Neutrons in the middle of the Atom and Electrons around the outside. Electrons have a negative charge, opposite to the Protons. Normally an Atom will have the same number of protons and electrons, so that its total charge is zero.
The Element below is an Atom of Lithium, which has 3 Protons, 4 Neutrons and 3 Electrons.
While electrons cannot be split into anything smaller, protons and neutrons are actually made of even smaller particles. They are both made from Quarks, a proton contains two Up Quarks and one Down Quark, and a neutron contains one Up Quark and two Down Quarks.
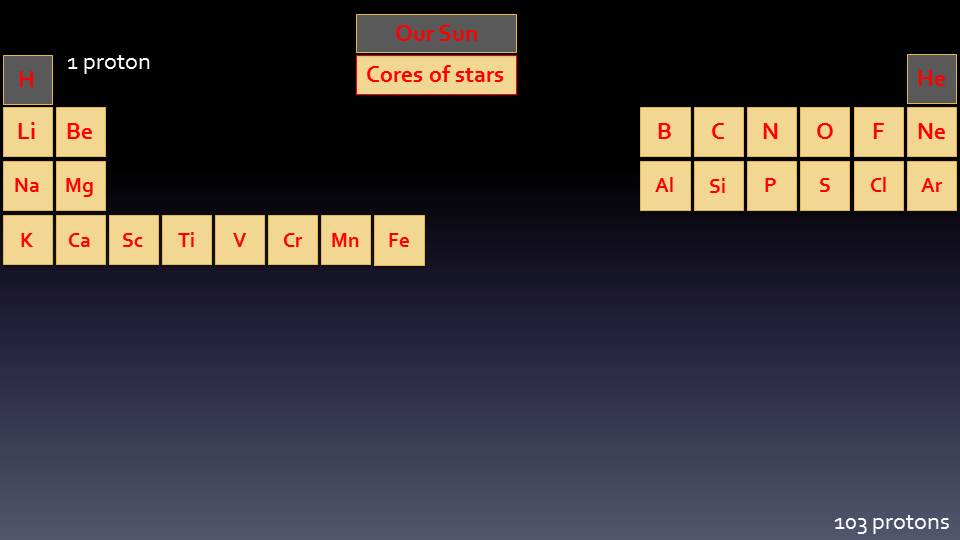
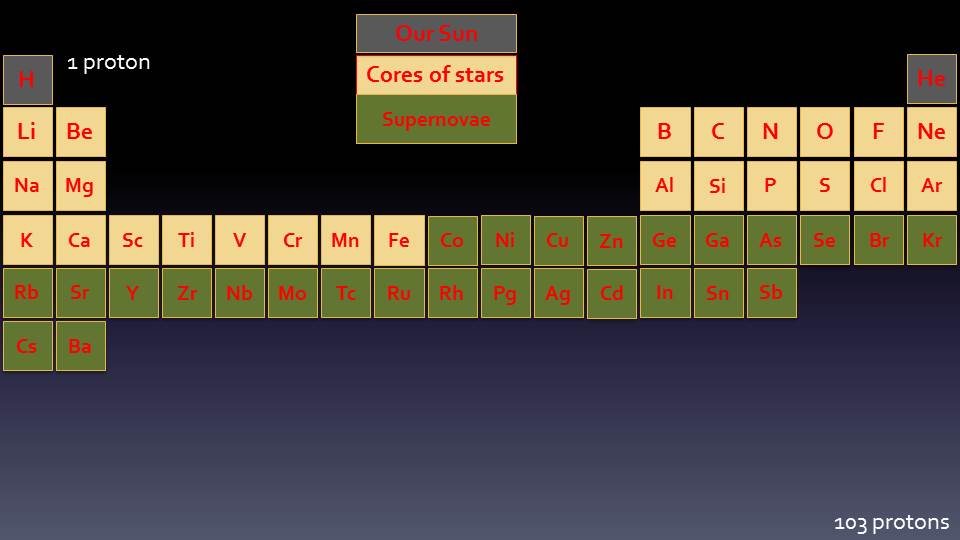
Periodic Table
The Periodic Table of the Elements shows all the different discovered Elements from the smallest to the biggest. Hydrogen has one Proton and is the first Element; Helium has two Protons and is next; and so on. All the Elements are placed in the table according to how many Protons they have.
It is called the Periodic Table because of the order and patterns that different Elements have. For example, all the elements in the very first column (called a “Period”) are metals that will explode when dropped into water; and the elements in the last period are all gases that will not have any chemical reactions. The Periodic Table has a very unique shape, which is based upon how the electrons are arranged around the outside of the atom.
Electrons do not simply orbit randomly around the nucleus, like the planets in the solar system. Instead they are arranged into Shells and Sub-Shells. Each row of the Periodic Table shows another Shell (or layer) of electrons and each block refers to a different Sub-Shell.
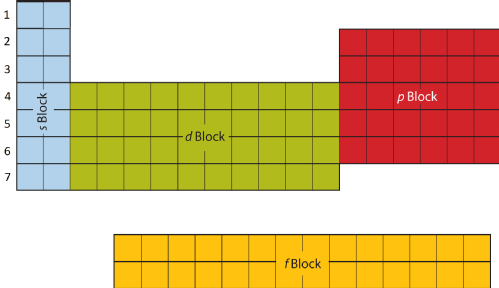
The Shells are numbered with 1 being the first shell closest to the nucleus. The Sub-Shells are given letters, where s is the first, p is second, then d, f and so on. Not every Shell contains every Sub-Shell, which leads to the overall shape of the Periodic Table. You can see that Shell 1 only has the s Sub-Shell, but Shell 2 has s and p.
We will see that all of the elements in the Periodic Table are formed in stars, in many different processes. The smallest elements are made in small stars, but to make bigger and bigger elements we need bigger stars, higher temperatures and the Biggest Bangs that the Universe can offer.
Nuclear Fusion
In a star Protons and Neutrons are added together to form larger and larger Elements. Sometimes a Proton will change to a Neutron, or the other way round, if there are too many of one type.
Stars are incredibly heavy - the sun is 300,000 times heavier than the earth. They are so heavy that all the atoms in the star are being pushed into the middle of the star by Gravity.
The incredible heat of a star is enough to remove all the Electrons from the atoms in the star. This makes a Plasma, where the positive nucleus has no negative electrons surrounding it. The positive nuclei repel each other, but the star’s strong Gravity pushes all of the nuclei close enough together for them to touch, and to Fuse together.
When two nuclei are forced together, they do not always bounce off one another. If there is enough energy, they are able to stick together to make one bigger atom. In this way stars make new Elements by joining smaller ones together. This process is called Nuclear Fusion.
When this happens, lots of heat energy is released, which is what makes stars so hot (the Sun is over 1 million degrees)!
Fusion in our Sun
The Sun is a simple star and there are only two Elements found in the sun. They are the simplest and smallest Elements: Hydrogen and Helium. A Hydrogen atom contains one Proton and zero Neutrons. When two Hydrogen nuclei collide, one of the Protons undergoes a type of radioactive decay called Beta-plus Decay, where a proton transforms into a neutron. This happens when there are too many protons in the nucleus, because the positive protons repel each other.
When two Protons fuse together, one of them decays into a Neutron. Our new nucleus now has one Proton and one Neutron. It is still Hydrogen, because there is still the same number of Protons, but it is a different type, or isotope, of Hydrogen. This bigger Hydrogen has the symbol 2H, showing that there are two particles in the nucleus.
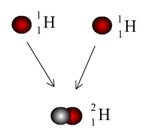
This process continues and more Hydrogen atoms are fused together, making a Helium isotope with two Protons and one Neutron – 3He. When two 3He atoms fuse together, it forms the most stable Helium isotope, with two Protons and two Neutrons – 4He.
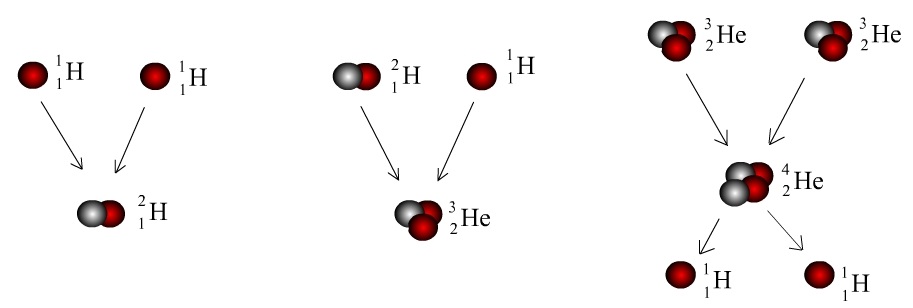
It is this exact Nuclear Fusion process that occurs in the Sun and makes it so hot. When two atoms are fused together, this releases an incredible amount of energy when they fuse. All the heat and energy in the Sun come from the creation of Helium from Hydrogen.
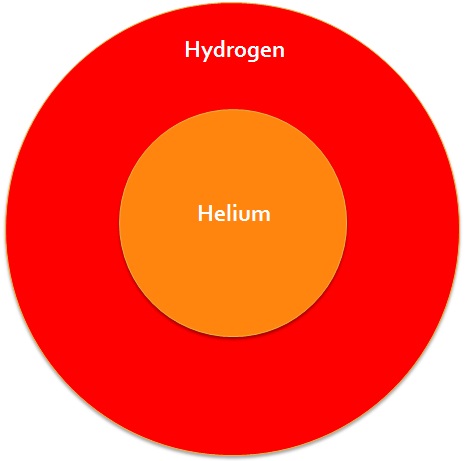
The Sun is not the same all the way through. In the middle where it is hottest, Nuclear Fusion takes place, converting Hydrogen into Helium. Around the outside it is not hot enough to form Helium (although still hundreds of thousands of degrees) and the Hydrogen waits to be fused together.
The Sun will take 8 billion years to turn all of its Hydrogen into Helium, and it is only about halfway through! Once it has used up all of its Hydrogen, the Sun will turn into a different type of star called a Red Giant.
So far we have seen how the first two elements are formed: Hydrogen is formed of one Proton, and Helium is made by combining Hydrogen atoms together in the centre of stars like the Sun.
We can show these on a Periodic Table, and see how much of it we have built up so far:

Heavier Stars, Heavier Elements
The Sun is only a little star compared to some of the other stars in the galaxy, even though compared to us on earth it seems enormous.
In much heavier stars, once all of its Hydrogen has been turned into Helium, they will start joining Helium atoms together to make bigger elements. These are the elements which make up all of life on earth i.e. Carbon, Oxygen and Nitrogen.
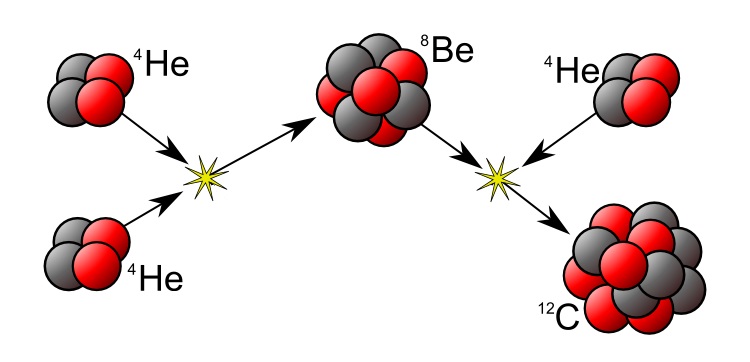
Making these elements releases lots and lots of energy, making these bigger stars even hotter than the Sun.
These new elements will be made in the middle of the star, causing layers of different elements to be formed. You can think of this a little bit like an onion, where each different layer represents another element that has been made.
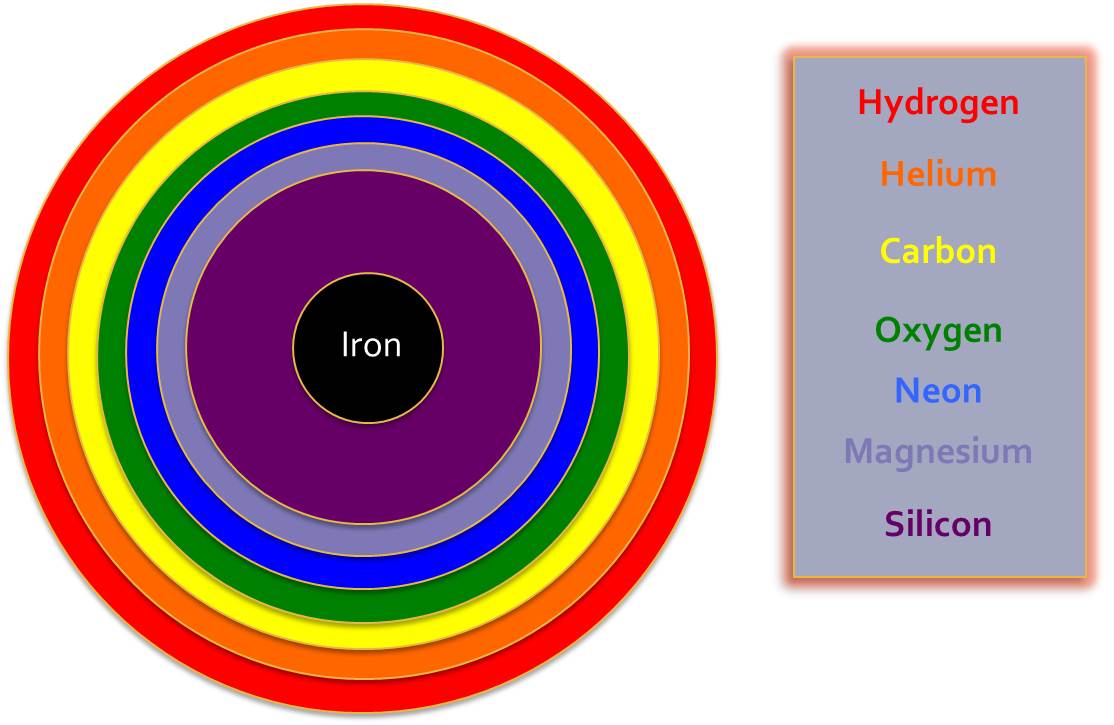
In the heaviest stars more Helium atoms can be added to Carbon to make bigger and bigger atoms. The biggest atom formed like this is Iron, which has 26 protons and 30 neutrons (56Fe). The new elements are made by joining the elements we already have to one another.
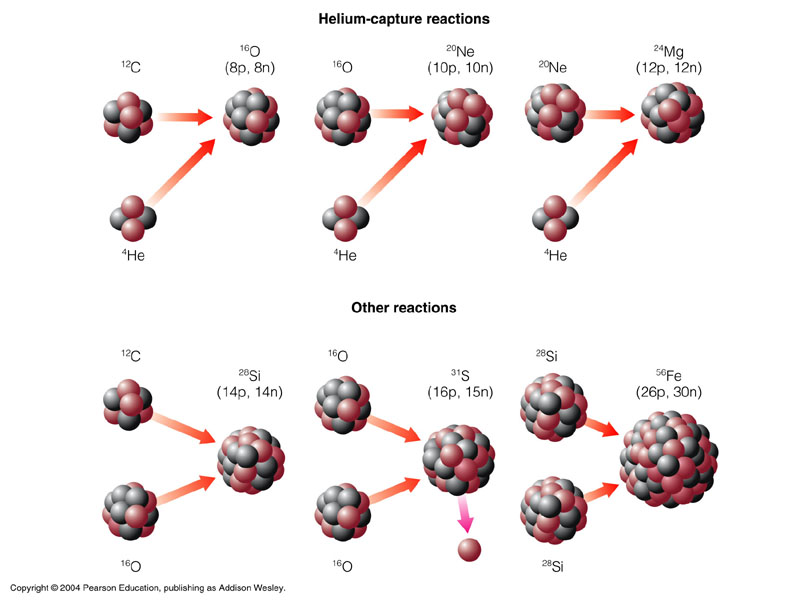
In larger stars we can start to form the next group of Elements, and these Elements are the most common ones on earth, are what forms the basis of all life and creates the earth’s core, crust and atmosphere. We can see where these new Elements fit into our Periodic Table:
Supernova
In stars there are two opposite forces that balance each other out while Nuclear Fusion is happening: Gravity and Pressure.
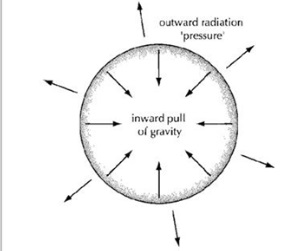
Gravity attracts masses towards one another. It pushes all the atoms into the centre of the star, making the star as small as possible. Stars are super heavy and therefore the Gravity is very strong.
Pressure is made by the temperature in the centre of the star. When atoms are heated, they move around faster and faster, and push outwards to take up more space (a thermometer works because the Mercury inside gets slightly bigger as it gets hotter). Atoms in a star are moving around very fast, pushing outwards as they heat up, making the star as big as possible.
While fusion is taking place, these two opposite forces balance each other out and nothing changes. But when a star runs out of atoms to fuse together, it stops creating heat. This means that there is not enough Pressure, and the Gravity makes the star collapse into the middle.
All of the atoms rushing into the middle cannot fit and bounce back from the centre. This makes the star explode, throwing its atoms back out into space. This is a Supernova!
During a Supernova even bigger elements can be created.
The huge temperature of a supernova explosion creates new elements in a new way. Instead of adding two small elements together, Neutrons are added to the atoms from the Supernova. If there are too many Neutrons in a nucleus, some of them will Decay into Protons, in a process called Beta-minus Decay. The creation of another Proton in the Nucleus means that it becomes a different Element.
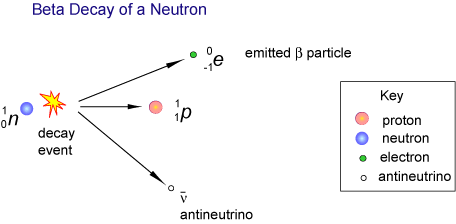
In a Supernova 80 Neutrons might be added to an atom in less than a second, creating elements twice as big.
In this way, Supernovae create over 25 new elements in the explosion of the star, including Copper, Silver and Bromine. The largest element made in a Supernova is Barium, which has 56 Protons and 82 Neutrons.
Supernova Remnants
When a Supernova explodes, we can look at the elements thrown out into space to see what had been made in the core of the star. Each element will have its own unique colour fingerprint, which can be detected by telescopes looking at the remains of Supernovae – Supernova Remnants.
Looking at the colours of the elements might seem strange initially, after all many elements look very similar. However each element will burn a different colour. The colour is based on the arrangement of the Electrons around the nucleus and is unique for every element.

We can look at the colours in a Supernova Remnant to see what elements were made inside the star before it exploded. Above is a tiny part of a Supernova with all of the elements shown. On the left are the elements separated out into their specific colours. This part of the Supernova Remnant contains three elements: Hydrogen (red), Oxygen (blue) and Sulphur (green).
The unique colours are given off when electrons around the nucleus are given more energy. Electrons orbit the nucleus at fixed distances and can move from one level to another. Electrons want to stay as close to the Nucleus as they can get, because their negative charge is attracted to the positive charge of the Protons in the Nucleus.
When given energy, an Electron can be excited to a higher level (further away from the nucleus). The Electron then falls back down to a lower energy state and, as it does so, energy is given out in the form of light.
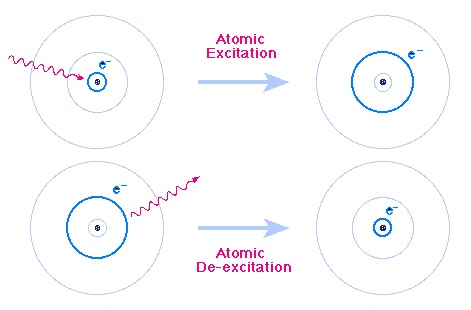
The colour of the light will have a very specific wavelength, depending on the energy levels of the higher and lower electron states. The difference in energy between the two levels will define what wavelength the emitted light will have.
In every element, the electrons orbit at different distances from the Nucleus, because the Electrons have different levels of attraction to the Protons in the Nucleus. More protons create a greater attraction, but having more electrons in the nucleus shields the outermost Electrons from the attractive pull.
Every element will have different positions that the Electrons can be in, different energy gaps between these positions, and therefore different colours associated with them. In the picture below you can see the colours associated with a group of metals when they are burnt. The colours are unique and allow them to be easily identified.

Five different metals can all be seen with very different flame colours. The metals used above are Lithium (Li), Strontium (Sr), Sodium (Na), Copper (Cu) and Potassium (K). The colours seen here would be exactly the same as seen in a supernova remnant. Sodium burns a very bright orange colour, which is the same colour as street lamps, because street lamps use Sodium metal in them to produce the light.
Neutron Star Mergers
So far we have seen how we make most of the elements that we know about on earth, including the most important and common elements. Carbon, Oxygen and Hydrogen make up most of our bodies; Iron makes up the core of the earth; and other elements like Calcium and Aluminium are used every day.
The very biggest elements like Gold and Uranium are very rare and made when two super heavy stars collide into one another. These super heavy stars are left over after a Supernova, and they are called Neutron Stars.
A Neutron Star is the densest object known to exist in the universe. Imagine the Sun, which is 300,000 times heavier than the earth, being squeezed down until it is only 10 miles across!
A Neutron Star is made almost entirely from Neutrons, one of our three building blocks that make up atoms. Around the outside of a Neutron Star there is a thin layer of heavy elements from before the supernova; for example, Copper, Silver and Barium.
When two Neutron Stars are formed near one another, they will gradually orbit closer and closer to each other until they eventually collide. When they collide, there is a very short moment (less than half a second) when the temperature is hot enough for hundreds of Neutrons to be added to the elements on the outside of the stars.
As we have seen before, when there are too many Neutrons in a Nucleus, they will undergo Beta-minus Decay and become Protons. In this way the very heaviest and rarest elements will be made.
Some of the elements formed are Radioactive Isotopes: these are super heavy nuclei which will decay into other elements. They do not decay by a Neutron turning into a Proton, but by splitting into two separate nuclei – Nuclear Fission.
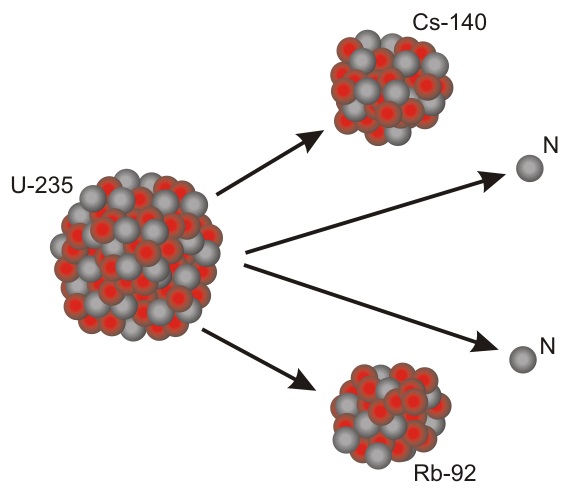
Although these elements will naturally decay and split into smaller elements, they will take a very long time to do so, sometimes billions of years. Uranium-235 (235U) is a Radioactive Isotope and is the fuel used in Nuclear power stations.
Neutron Star Mergers also create a number of other heavy elements, such as Gold, Platinum and Lead.
The force and power of two incredibly heavy, dense stars colliding mean that Neutron Star Mergers are the biggest and brightest explosions in the entire universe. For the few seconds that they happen, they are brighter than all the other stars in the universe put together.
These are the Biggest Bangs.
