Transient Electronic Absorption Spectroscopy
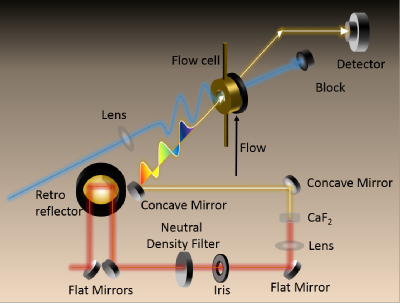
Transient Electronic Absorption Spectroscopy is the time resolved study of the absorption spectrum of a sample as a function of time, following excitation from a femtosecond laser pulse. The excitation pulse can be tuned to any wavelength in the range ~235-1600 nm using an optical parametric amplifier (TOPAS), seeded with a 1 kHz, 40 fs, 800 nm pulse (Ti:Sapphire Spitfire ACE) to create pulse powers of around 10 uJ / pulse. The probe pulse is a white light continuum, created by focussing the fundemental 800 nm laser onto a CaF2 window, producing light of ~330-730 nm. The excitation pulse pases through the sample, refreshed for each pulse pair by use of either a flow cell or gravity jet apparatus, to prepare the sample in some electronically excited state. The white light probe then passes through the excited sample after some delay time, which can be varied by way of a gold retroreflector mounted on a delay stage which increases the path length of the 800 nm beam creating the probe pulse. The white light is mointored with a spectrometer (Avantes), which also takes dark backgrounds and unpumped spectra to find the difference in absorption following excitation. By taking a number of these difference asborption spectra over time, a picture of the dynamics of the excited state can be revealed.
The stage that generates the delay between pump and probe pulses has a travel range of 500 mm with a resolution of 5 microns, reproducible to within 0.5 microns. This corresponds to a maximum temporal window of 3.3 nanoseconds at a resolution of 17 femtoseconds. The Avantes spectrometer has a resultion of 1.2 nm, with an intergration time of 0.85 miliseconds, allowing a maximum sample rate of 1.1 kHz, thus fully sampling the 1 kHz laser. It has a 900 nm spectral range, though typically only ~400 nm is utilised in the TEAS experiment. It can store up to 1254 spectra to RAM at any given time.

