Eliminating sleeping sickness in Africa
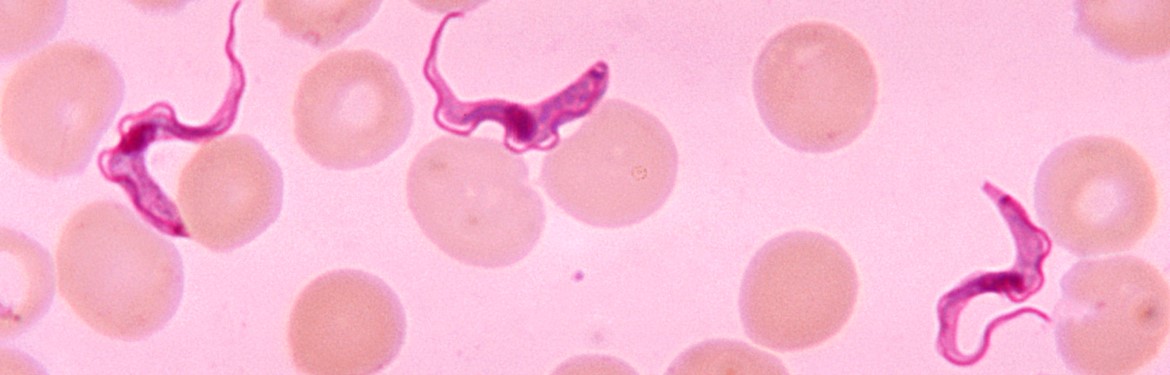
Eliminating sleeping sickness in Africa
Informing policy and healthcare interventions to help save lives
At the height of the most recent sleeping sickness epidemic, 37,385 cases were reported in Africa leading to thousands of deaths and impacting many lives. In 2022 there were estimated to be 42 million people living at risk of contracting sleeping sickness across West and Central Africa, however, internationally coordinated efforts in the past two decades have led to a substantial decline in the number of cases.
Professor Kat Rock and her team of modellers and health economists at the University of Warwick are working alongside national sleeping sickness control programmes and their partners to provide decision support around resource use and intervention strategy planning as part of the global support for achieving the World Health Organization’s (WHO) target of eliminating the transmission of sleeping sickness by 2030.
The challenge
Despite significant progress towards the elimination of sleeping sickness, many challenges remain. The parasite, responsible for around 95% of cases, known as gambiense human African trypanosomiasis (gHAT) is transmitted to humans through the bite of an infected tsetse (an African bloodsucking fly). If left untreated during the early stages of the disease, the parasite will cross the blood-brain barrier and invade the central nervous system. This leads to severe disease symptoms including sleep disruption, confusion, lethargy, convulsions and eventually death.
Although disease interventions have been successful in reducing cases over the past 20 years, with some countries having validated elimination of the disease as a public health problem, different challenges emerge as we move closer to elimination.
Our approach
Using bespoke mathematical models, the Warwick team, led by Professor Kat Rock, assessed past transmission and used this to make predictions for the future under a range of specified strategies. These have been used to provide recommendations for cost-effective ways forward at a regional level.
The models were developed to reflect the epidemiological situation in specified countries at different stages of disease elimination. Initial work focused on the Democratic Republic of Congo (DRC) which currently has around 70% of global cases.
The model was then used to estimate the impact of current control strategies and when transmission may end under different elimination plans. These analyses identified that additional strategies, including tsetse control and/or improved screening, may help eliminate the disease in the DRC.
The model has also been used to perform similar assessments in Chad, identifying that transmission was already interrupted in 2015 due to the intensification of interventions and that careful monitoring is now required to ensure that the desired outcome is achieved.
Our impact
The research examined medical interventions required to meet transmission elimination in the DRC by 2030. The findings revealed new priority health zones where large-scale insect control would be needed to help eliminate transmission. This contributed to the DRC’s national sleeping sickness programme’s decision to implement scale-up vector control between 2019 and 2022.
In Chad, implementing the Warwick model in the country’s Mandoul region made it possible to quantify the effect of screening and insect control interventions. These interventions saw cases reduce from 715 in 2002, to fewer than 20 cases in 2018-19. Vector control was first implemented in 2014 in the swampy Mandoul region, which has an approximate population of 40,000, and where most gHAT cases occurred between 2000 and 2013. Adaptation of the Warwick model to Mandoul made it possible to quantify the effect of interventions (both improved passive screening and vector control). Modelling suggested that these combined measures led to a 72% reduction in transmission.
Continuing implementation of these interventions saw cases gradually reduce from 715 in 2002 to fewer than 20 cases in 2018 and 2019. The Warwick modelling for Chad provided important validation that the approach in Mandoul was an optimal strategy.
The HAT MEPP team have continued to work closely with policymakers and other stakeholders to undertake assessments and support decision making. For example, the research has:
- Assessed the likely impact of interruptions to sleeping sickness interventions due to other diseases (like COVID-19 or Ebola), loss of funding or political instability
-
Supported planning for “exploratory screening” to evaluate if local elimination has occurred in places reporting no recent cases.

A health facility in Bonon, Cóte d’Ivoire.
The HAT MEPP team continue to work with national programmes in Chad, the Democratic Republic of Congo, Guinea, Côte d’Ivoire and Uganda to support their public health activities.
The research was funded by the Bill and Melinda Gates Foundation.
Top image credit: CDC/Dr. Myron G. Schultz.

What is research impact?
Discover why it matters
More impact stories
Explore other work from Mathematics and Statistics at Warwick
