Templates for Talks, Posters and Reports
This page will contain links to group presentation templates and useful items such as University Logos etc.
It will also contain templates for writing reports including those for experimental sections, chem draw figure settings etc.
Matt's paper writing guidelines:
1 - All figures to be made in origin (excel is rubbish) and the pasted (using Ctrl+J then Ctrl+V - no other combination works) into a powerpoint slide. This way I can edit the data directly.
2 - READ instructions. IF the title can only be 20 words long, it should not be more than 20 words long. If the author list using full names (e..g Matthew I. Gibson) do not use abbrevations (M.I.Gibson).
3 - Citation style - check the citation style by looking at current papers in the journal we send to. Do NOT trust the endnote style file to be correct. You normally have to edit these.
4 - Do no mess up template files. If they have a specific font (e.g. arial) do not paste text as Calibri into it (Use paste and match formatting options)
5 - Never copy and paste figures into a word file. Use ‘insert picture’ function to insert a .tiff file
6 - All papers need a graphical abstract. Preferably these should not be rubbish.
All papers should start with an outline - i.e. all the data and figures plotted and then we will decide which to use. This must happen before you start writing it. Don't bother starting to write the text until you have sat down with me to discuss what is going in the paper (to avoid wasting your time).
Matt's Graph Guidelines:
1 - Use Origin
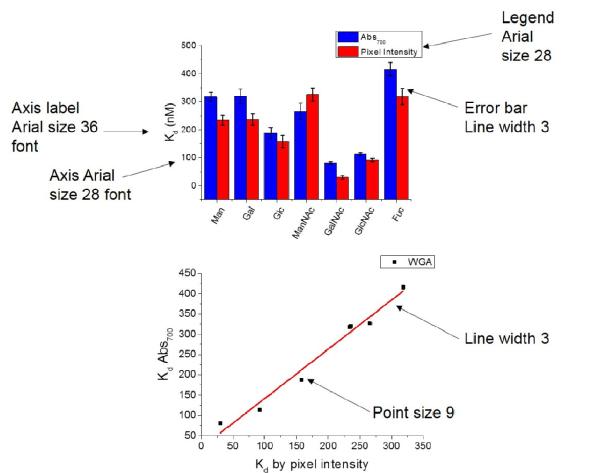
2 - Axis should be Arial size 28
3 - Axis labels should be Arial 36
4 - Line widths should be 3
5 - Use black, red, blue, olive in that order
6 - error bars line width 3
7 - don't resize or do anything, just Ctrl+J, Ctrl+V into powerpoint
8 - if your figures do not look like those we have published before, they are probably wrong
9 - microscope images need a scale bar that is READABLE
10 - See examples below:
Posters: Here is an example of a poster. Fancy graphics are bad and do not improve anything. The aim is to have a brand which features the warwick logo on every poster but is also unique to our lab.![]()
Poster Template (new branding) is here ![]() Example poster in new template
Example poster in new template ![]()
Making images for webpage to advertise new papers
Use TOC image - copy into powerpoint and apply text (title, citation) using Franklin Gother Book, size 14 as the font.
Use image software (such as GIMP) to generate JPEG - total size of file should be below 100 KB
How to Report Polymer RTP equipment in papers
WHEN USING POLYMER RTP EQUIPMENT PLEASE INCLUDE THIS STATEMENT
IN YOUR ACKNOWLEDGMENTS.
We are grateful for the Polymer Characterisation RTP for providing use of the following
equipment: (ADD INSTRUMENTS USED)
GPC/SEC
CHCl 3
Agilent Infinity II MDS instrument equipped with differential refractive index (DRI), viscometry (VS) and dual angle light scatter (LS) detectors. The system was equipped with 2 x PLgel Mixed C columns (300 x 7.5 mm) and a PLgel 5 µm guard column. The eluent is CHCl 3 with 2 % TEA (triethylamine) additive (Check whether additive has been used in a given month). Samples were run at 1ml/min at 30’C. Poly(methyl methacrylate), and polystyrene standards (Agilent EasyVials) were used for calibration. Ethanol was added as a flow rate marker. Analyte samples were filtered through a GVHP membrane with 0.22 μm pore size before injection. Respectively, experimental molar mass (Mn, SEC ) and dispersity (Đ) values of synthesized polymers were determined by conventional calibration using Agilent GPC/SEC software.
DMF
Agilent 390-LC MDS instrument equipped with differential refractive index (DRI), viscometry (VS), dual angle light scatter (LS) and UV detectors. The system was equipped with 2 x PLgel Mixed D columns (300 x 7.5 mm) and a PLgel 5 µm guard column. The eluent is DMF with 5 mmol NH 4 BF 4 additive. Samples were run at 1ml/min at 50’C. Poly(methyl methacrylate) standards (Agilent EasyVials) were used for calibration between 955,000 – 550 gmol -1 . Analyte samples were filtered through a nylon membrane with 0.22 μm pore size before injection. Respectively, experimental molar mass (Mn, SEC ) and dispersity (Đ) values of synthesized polymers were determined by conventional calibration and universal calibration using Agilent GPC/SEC software.
THF
Agilent 390-LC MDS instrument equipped with differential refractive index (DRI), viscometry (VS), dual angle light scatter (LS) and dual wavelength UV detectors. The system was equipped with 2 x PLgel Mixed C columns (300 x 7.5 mm) and a PLgel 5 µm guard column. The eluent is THF with 2 % TEA (triethylamine) and 0.01 % BHT (butylated hydroxytoluene) additives. Samples were run at 1ml/min at 30’C. Poly(methyl methacrylate) and polystyrene standards (Agilent EasyVials) were used for calibration. Analyte samples were filtered through a GVHP membrane with 0.22 μm pore size before injection. Respectively, experimental molar mass (Mn, SEC ) and dispersity (Đ) values of synthesized polymers were determined by conventional calibration using Agilent GPC/SEC software.
Aqueous
Agilent Technologies Infinity 1260 MDS instrument equipped with a differential refractive index (DRI), light scattering (LS) and viscometry (VS) and UV detectors. The column set used were Tosoh TSKGel GPWXL *2. The mobile phase used was 0.1 M NaNO 3 . Columnoven and detector temperatures were regulated to 40°C, flow rate 1 mL/min (These change regularly – please check). Poly(ethyleneoxide) standards (Agilent EasyVials) were used for calibration between 1,368,000 – 106 gmol -1 . Analyte samples were filtered through a hydrophilic GVWP membrane with 0.22 μm pore size before injection. Respectively, experimental molar mass (Mn, SEC ) and dispersity (Đ) values of synthesized polymers were determined by conventional calibration using Agilent GPC/SEC software.
PL50 DMF
Agilent PL50 instrument equipped with differential refractive index (DRI) and UV detectors. The system was equipped with 2 x PolarGel M columns (300 x 7.5 mm) and a PolarGel 5 µm guard column. The eluent is DMF with 0.1 % LiBr additive. Samples were run at 1ml/min at 50’C. Poly(methyl methacrylate) standards (Agilent EasyVials) were used for calibration. Analyte samples were filtered through a nylon membrane with 0.22 μm pore size before injection. Respectively, experimental molar mass (Mn, SEC ) and dispersity (Đ) values of synthesized polymers were determined by conventional calibration using Agilent GPC/SEC
software.
PL220
Agilent PL220 instrument equipped with differential refractive index (DRI), viscometry (VS) and dual angle light scatter (LS 90 + 15) detectors. The system was equipped with 2 x PLgel Olexis columns (300 x 7.5 mm) and a 10 µm guard column. The mobile phase was TCB with 250 PPM BHT (butylated hydroxytoluene) additive. Samples were run at 1 ml/min at 160’C. Polystyrene standards (Agilent EasyVials) were used to create a third order calibration.Analyte samples were filtered through a stainless steel frit with 10 μm pore size before injection. Respectively, experimental molar mass (Mn, SEC ) and dispersity (Đ) values of synthesized polymers were determined by conventional calibration using Agilent GPC/SEC software.
PL50 Aqueous
Agilent PL50 instrument equipped with differential refractive index (DRI) detector. The system was equipped with 2 x Aquagel H columns (300 x 7.5 mm) and an Aquagel 5 µm guard column. 80:20 0.1 M NaNO 3(aq) :methanol. Samples were run at 1ml/min at 35’C. Poly(ethylene oxide) standards (Agilent EasyVials) were used for calibration. Analyte samples were filtered through a membrane with 0.45 μm pore size before injection. Respectively, experimental molar mass (Mn, SEC) and dispersity (Đ) values of synthesized polymers were determined by conventional calibration using Agilent GPC/SEC software.
The following instrument conditions are much more user dependent, so pay attention to what
you have done.
Thermal Analysis
DSC
Mettler-Toledo DSC1 with autosampler. Mention gas used, heating rate, heating range and pan type.
TGA
Mettler-Toledo TGA with autosampler. Mention gas used, heating rate, heating range and pan type.
TGAMS
Mettler-Toledo TGA with autosampler and Hiden Mass Spectrometer. Mention gas used, heating rate, heating range and pan type. For MS mention scan range and rate.
Particle Sizing
DLS
DLS measurements were carried out on a Malvern Zetasizer in (dispersant), for X runs.
Laser Diffraction
Laser diffraction measurements were carried out on a Malvern Mastersizer 2000 in (dry or wet) mode (if wet dispersion mention dispersant). Mentioned dispersion method, i.e. in situ sonication and stirring.
Drop Shape Analyser
DSA measurements (static contact angle/pendant drop) were carried out on a Kruss DSA 1
Example viva questions ![]() for anyone having to talk about their work.
for anyone having to talk about their work.
ACD labs NMR software tutorial ![]()
http://www.acdlabs.com/resources/knowledgebase/movies/
Above link is also useful for learning how to use ACD labs NMR software
Talk template slides
Talk template, new branding, with updated acknowledgments slide ![]()
Useful slides containing images of cell membranes and intracellular contents for poster and presentation figures.
Manual for the Edwards Pumps we use![]()
UV Temperature Controller Instructions
Christmas shutdown form 1.07 fridge
Christmas shutdown form 1.05 fridge
Christmas shutdown form 1.05 freezer
Christmas shutdown form 1.05 MilliQ
Christmas shutdown form 1.07 freezer
Powerpoint containg the image of the analytical lab equipment, please update this and insert a new image to the webpage if you get any new equipment ![]()
Useful Links
SugarBind DB (Pathogen Sugar Binding Database) http://sugarbind.expasy.org/
"SugarBindDB, a resource of glycan-mediated host–pathogen interactions" - Paper explaining how to use the database
Thermo Fisher Protein Expression Handbook
Useful Contacts
OCTET: Gail Calvert, Field Applications Scientist, UK and Ireland ForteBio
Mobile: +44 (0) 7870 263241
E-mail: gail_calvert@pall.com
Useful people in SLS:
Erich Ratamero- Research Fellow in Computing/Image Analysis.
Erich is here to provide computing support for our research groups; largely directed towards image analysis, storage and automation needs. Erich is located in the open plan office space on the ground floor of the MCB building and is managed by Steve Royle. In due course, Steve will be providing details of how you can access support from Erich and will also be able to answer any questions you may have.
Tauqeer Alam- Research Fellow in Bioinformatics.
